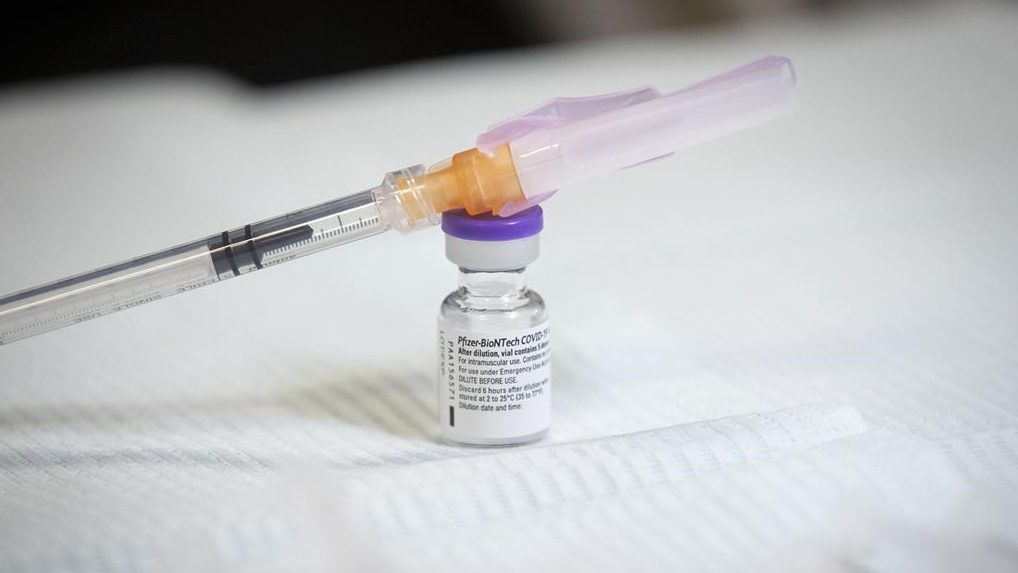
Health Canada has approved the Pfizer-BioNTech COVID-19 vaccine for children 12 to 15 years old.
Posted May 06, 2021, 05:04AM EDT
Health Canada has approved the Pfizer-BioNTech COVID-19 vaccine for children 12 to 15 years old.
The vaccine was initially approved for use in those aged 16 and older in December, and Health Canada received an application from Pfizer to expand the age threshold on April 16 of this year.
“After completing a thorough and independent scientific review of the evidence, the department determined that this vaccine is safe and effective when used in this younger age group,” Health Canada’s chief medical adviser Dr. Supriya Sharma said on Wednesday.
A trial of more than 2,200 youth in that age group in the United States recorded no cases of COVID-19 among vaccinated kids. The trial used the same size doses, and the same two-doses requirement, as the vaccine for adults.
Sharma said the efficacy of the Pfizer vaccine for that age group was 100 per cent after the second dose.
“This is the first vaccine authorized in Canada for the prevention of COVID-19 in children and marks a significant milestone in Canada’s fight against the pandemic,” she said.
Sharma said about one-fifth of all cases of COVID-19 in Canada have occurred in children and teenagers, and having a vaccine for them is a critical part of Canada’s plan.
“While younger people are less likely to experience serious cases of COVID-19, having access to a safe and effective vaccine will help control the disease’s spread to their family and friends, some of whom may be at higher risk of complications,” she said.
Health Canada said Wednesday that the updated approval is effective immediately, so if provinces choose to, they could start giving the shot to kids as young as 12.
Alberta Premier Jason Kenney was the first to announce that starting on Monday, his hard-hit province would make vaccines available to everyone aged 12 and up.
The news comes the day after high COVID-19 transmission rates forced the closure of schools in Alberta.
Manitoba followed suit shortly afterward on Wednesday, saying it aims to have those 12 and up eligible to book a vaccine by May 21.
Ontario Minister of Health Christine Elliott said the province was also “actively” working on a plan to vaccinate children aged 12 and older, but she did not provide a firm timeline for that plan.
“We want to make sure that our young people are protected from COVID as well,” Elliott said. “We’ve already been in conversations, discussions with the Minister of Education, to make sure that we can start as soon as possible.”
Elliott said the plan could see children aged 12 and older offered their first dose of a COVID-19 vaccine in schools, with a second dose given before the new school year begins in September.
She added that the province was also working to ensure education workers are able to get a second shot of a COVID-19 vaccine before September.
All schools are currently teaching classes online as the province remains under a stay-at-home order imposed due to high COVID-19 rates.
While now authorized for use for those aged 12-plus, Sharma notes trials are underway from numerous drug-makers to have COVID-19 vaccines approved for children as young as six months.
Pfizer CEO Albert Bourla said Wednesday the company expects to have data on trials in kids between two and 11 years old in time to apply for authorization in the United States in September.
The company has generally applied to Canada for approval around the same time but in this case Canada is ahead of the U.S. The U.S. Food and Drug Administration expects to authorize the vaccine for 12 to 15 year olds next week.
Health Canada maintains advice on taking offered vaccine
Earlier this week, the National Advisory Committee on Immunization (NACI) said given the risk of blood clots from AstraZeneca and Johnson and Johnson, people who are at lower risk of contracting COVID-19, or low risk of severe illness from it, can choose to wait for one of the mRNA vaccines from Pfizer or Moderna.
Sharma did not directly comment on NACI’s advice, but said every vaccine in Canada has been authorized because it is safe and effective.
She also said she still stands behind the advice to take the first vaccine you’re offered, as soon as you’re offered it.
Sharma said the risk of a new vaccine-induced blood clotting syndrome is extremely low, and for many Canadians there is a big benefit to getting vaccinated as soon as they can.
She said if you have access to any of the vaccine options at the same time, “absolutely there may be an advantage” to going with the mRNA vaccines from Pfizer-BioNTech or Moderna because they don’t carry any risk of blood clots.
But if you have to wait for Pfizer or Moderna and can get AstraZeneca now, getting immunized now is a good choice, she said, noting it takes at least two weeks for a vaccine’s full effect to take place.
More than a third of Canadians have received at least one dose of a COVID-19 vaccine, and Canada is on track to receive at least 10-million doses this month alone.
With files from Cormac Mac Sweeney